BIOSENSORS, MOTOR PROTEINS AND MORE
MARTIN WEBB
SCIENCE
Home > Research > Small G Proteins
SMALL G PROTEINS
INTRODUCTION
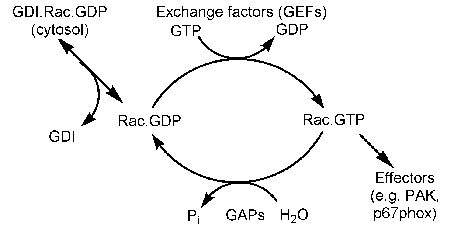
Small G proteins couple GTP hydrolysis to a molecular switch, forming part of many signaling pathways and various other cell functions, such as in secretion.
Although aimed at fundamental and general questions about this superfamily, research particularly focused on two small G proteins, Ras and Rac, to understand how the GTP hydrolysis cycle is coupled to this switching function.
Most share the features of interacting with specific GTPase activating proteins (GAPs), so deactiviting, and GEFs activating the protein, by controlling the nucleotide bound (GTP or GDP).
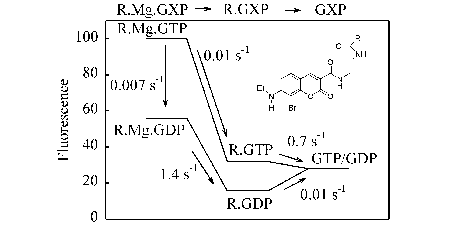
As a pre-requisite for studies with interacting proteins, the chemical and kinetic mechanism of the GTPase cycle of Ras was elucidated
I would like to acknowledge and thank all the people from my lab and elsewhere, who contributed to this work. Their names are on the cited publications.
Ras GTPase MECHANISM
A combination of techniques was used, including fluorescent nucleotide analogues and rapid reaction techniques showing a cleavage step that was essentially irreversible. (Ref 32, 38, 43)
The possibility of a phosphoenzyme intermediate was excluded, using the stereochemical technique we developed. (Ref 34)
In a collaborative project, Raman spectroscopy using guanine nucleotides, specifically substituted with oxygen-18, provided important, complementary evidence for in-line displacement of GDP by water with the GTP state having partial transition state properties. (Ref 59, 62)
A feature of the small G proteins is the very slow hydrolysis and nucleotide exchange in absence of extra protein factors. This feature was investigated using our fluorescent GTP analogues, outlined above. One particular analogue, when bound to small G proteins, Ras or Rac, showed sensitivity of its fluorescence to Mg2+ in the binding site. This extra signal then allowed demonstration that the mechanism of dissociation is obligatory by release of metal ion and nucleotide, even at normal Mg concentrations. (Ref 77)
RAC AND GDI
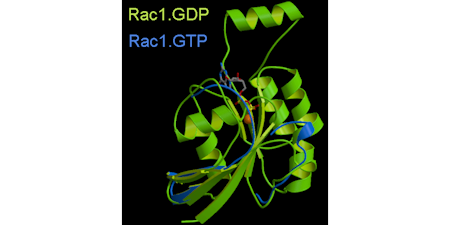
Rac1 belong to a different sub-family to Ras. We were involved in the structural determination of this protein, so the first structures for this sub-family. It shows an "extra" helix, that relates to interactions with other proteins , such as GDI
In the cell it is occluded by interaction with this protein GDI. We developed a method to study this interaction and so quantify the importance of this occlusion.
GAPs
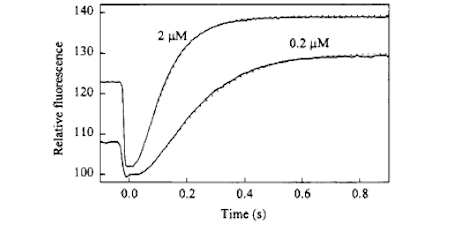
GAPs (GTPase activating proteins) effectively switch off small G proteins from the active GTP state, which otherwise have a very slow GTPase activity. The development of the phosphate biosensor, outlined above, provided new ways to examine the mechanism of the GTP hydrolysis cycle and, in turn, how the nucleotide state of Ras affects its interaction with the appropriate GAP.
By developing a fluorescence technique to determine the affinity, it was demonstrated that some oncogenic mutants of Ras with impaired GTP hydrolysis function, fully maintained their interaction with GAPs in the GTP state, but the latter was unable to catalyse the GTP hydrolysis with these mutants. While cleavage itself is accelerated by GAP, Pi release is associated with the transition from active to inactive state and the change in affinity for GAP.